GenCure’s 6,700 SF cleanroom production space is designed to be compliant with U.S., European and Japanese regulations for cell and cell-based biologics.
Cleanrooms are sized for a wide range of production volumes up to 250L scale bioreactors. Equipment in the cleanrooms mirror the equipment in the Process Development Lab, reducing variability as you move into clinical manufacturing.


In an effort to support Advanced Therapies manufacturing, GenCure’s technical capabilities include cell isolation and expansion of genetically modified cells.
GenCure’s cell manufacturing facilities are designed for clinical and commercial cGMP processing. The facilities have four Grade B cell culture processing suites supported by a Grade B purification suite, Grade C solution preparation suite, and a Grade C storage suite with a Grade D glass wash and autoclave suite. All of the suites are connected by a Grade C entry corridor and a Grade D exit corridor, allowing for unidirectional flow of materials and personnel.
GenCure’s cell expansion equipment includes:

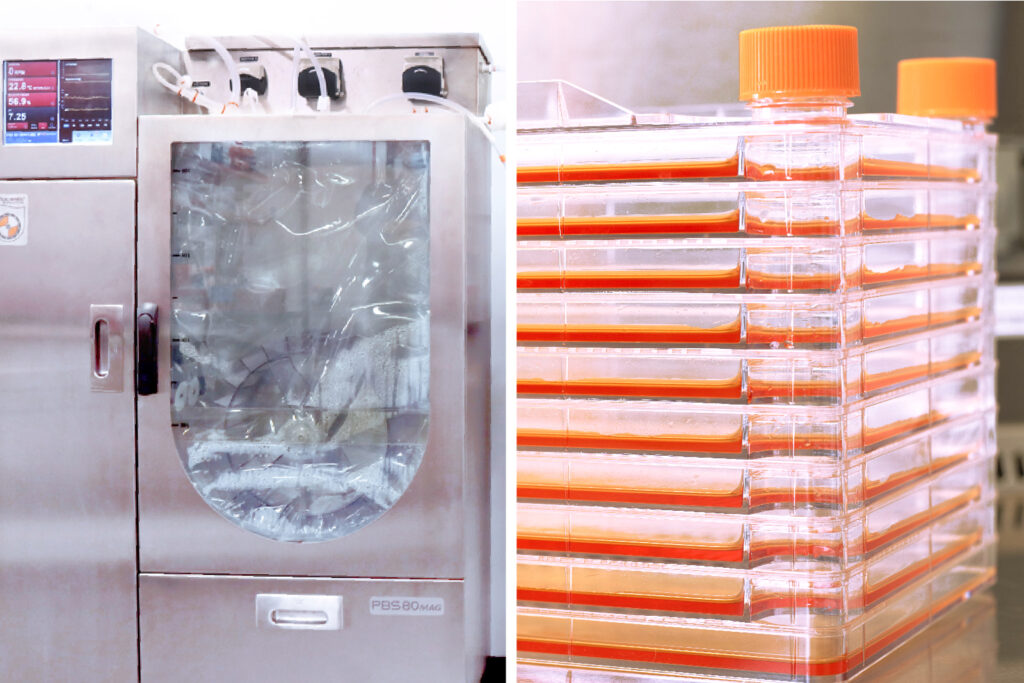
GenCure has the capacity to harvest cells or other products by standard centrifugation and filtration techniques. The team can wash and concentrate cellular derived products by ultrafiltration or diafiltration. At current capacity, the team can perform tangential flow filtration from 3L-80L, which scales accordingly with vertical wheel bioreactor systems. Final product filtration can be performed in-line, creating a closed sterile system that is optimized for cell therapy products.
The team’s equipment includes:

GenCure’s cleanrooms include a Grade B Fill/Finish suite suitable for clinical fills with the flexibility for manual fills or automated fills via the Terumo FiniaTM or Sartorius Fill ItTM.

GenCure has multiple LN2 and -80°C freezers located at both of its locations for client flexibility and business continuity. Storage facilities are temperature-monitored and locked with controlled access. The LN2 freezers are currently supported by dewars, but construction is underway to provide hard-piped bulk LN2 supply with both types of freezers on a back-up power supply to ensure all products are safely stored.
Scroll through for a virtual tour of the biomanufacturing facility.
